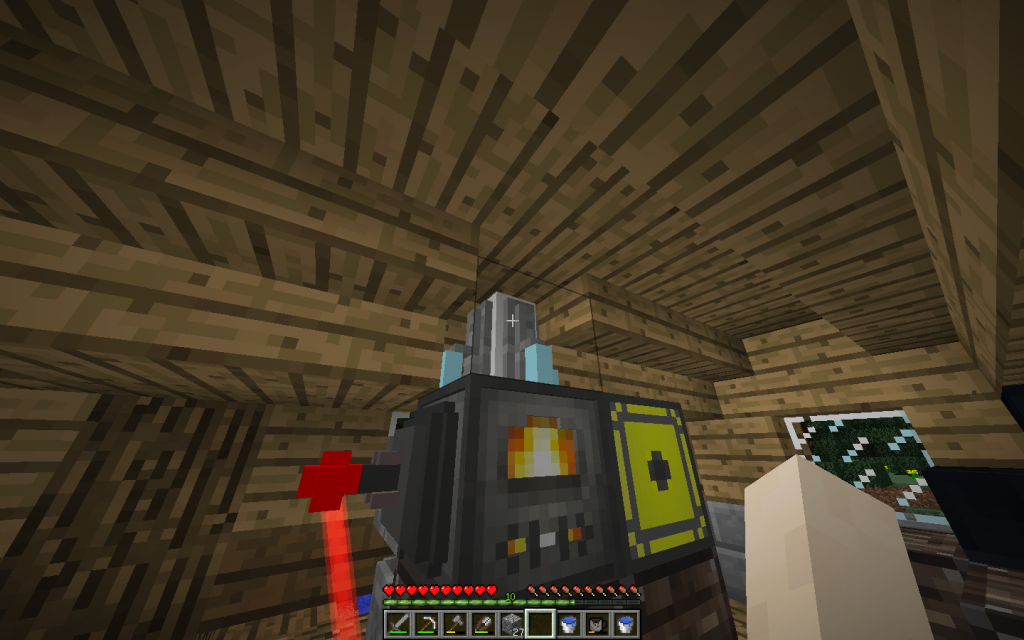
Powder of Silicon with strong heating burns into Silicon Dioxide (pages 446-448, 877-878, 928 of a chemical textbook).
Si + O2 = SiO2 + Q
The formula describes the chemical reaction, on the left side of the substance before the reaction. On the right side of the substance after the reaction. Such reactions can be carried out using our Chemical Combiner.
Q is the heat energy released during combustion, the wave of which causes the molecules surrounding the combustion source to move faster. Man perceives burning as light and heat.
- SETUP NEW MINECRAFT CLIENT FOR MINECRAFTFORGE 1.12 ON YOUR COMPUTER
- MINECHEM v6
- ALCHEMISTRY V 0.6.1 & ACTUALLY ADDITIONS
- DIAMOND
- NETHER FORTRESSES
- CHEMICAL MACHINES
- CELLULOSE
- MEDIUM STORAGE CRATE
- SUCROSE
- PROTEIN
- SILICON
- SILICON DIOXIDE
Chemistry Zumdahl, 7 ed. Houghton Mifflin Company Boston New York
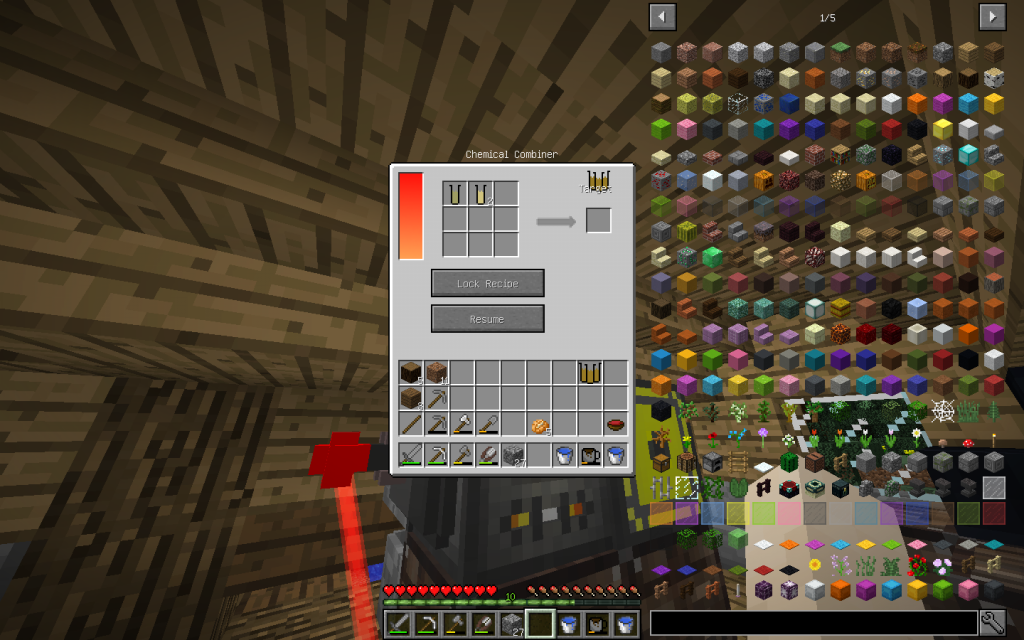
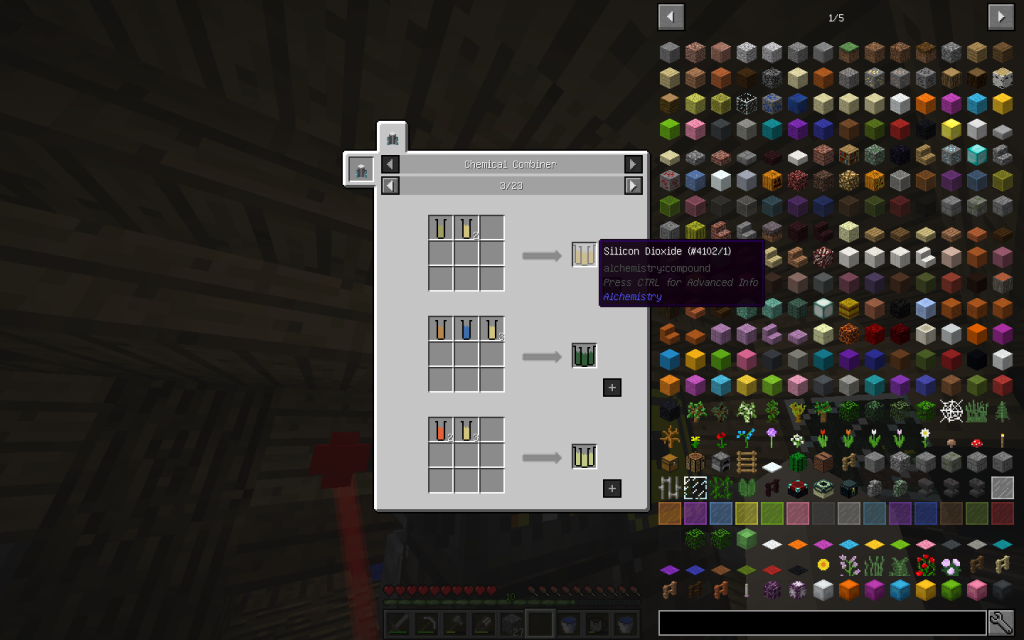
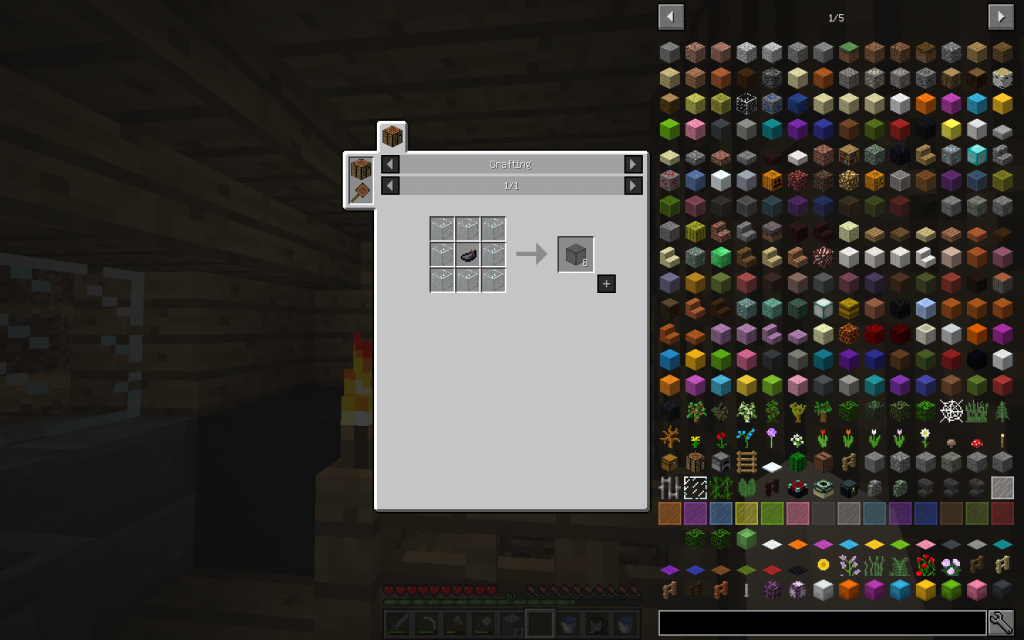
All elements from the right part are laid in the matrix on the left of the Chemical Combiner in accordance with the recipe. And in the square to the right we get the combined substance.
To start the recipe, the Resume button should be in the Pause state, and the Unlock Recipe button in the Lock Recipe state. If you click to the arrow, when the input components are not already placed, you can see the complete list of recipes for the Chemical Combiner.
The amount of the final substance in moles must be in balance with the input substances. In accordance with the law of chemical conservation of the masses, which was first discovered by the Russian scientist Lomonosov – the number of each type of atoms in the formula on the left must coincide with the number of atoms in the equation on the right.
Silica
Silicone Dioxide crystal with a melting point of + 1713 … + 1728 ° C, very hard and durable. Has no color.
In nature, silicon occurs in the form of silica – compounds based on silicon_dioxide SiO2. The main minerals and rocks formed by silicon dioxide are sand (river and quartz), quartz and quartzites, flint, feldspars.
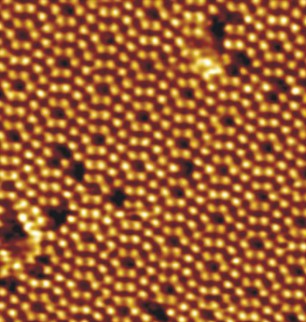
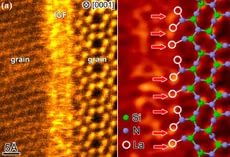
Silicone Dioxide Grating
(pages 446-448, 877-878, 928 of a chemical textbook)
The silicon atom is larger than the carbon atom, so around it, 4 atoms of Oxygen are quietly arranged, each with a separate bond. Therefore, unlike Carbon (which creates a molecular lattice with weak O = C = O bonds), Silicon forms an atomic lattice with the help of pi-bonds. Each atom has 4 or 3 nearest neighbors. Consequently, the 3 connection in the case of 3 neighbors in the plane goes up or down. In hexagons alternate atoms with 3 and 4 bonds in the plane.
It is possible to assume with confidence that those atoms with 4 bonds each have a horizontal position in the plane of the orbitals.
For others, the form of the orbitals is more elongated toward the remote atom. We can assume that it is on the layer below.
On average, every 2 atoms go up or down.
Therefore, silica is a solid and very refractory substance. In nature, Silica is found in the form of inclusions in granites and other rocks. Such inclusions appear on the rock fragments as pieces of fused glass.
What is the relationship of the molecular lattice of Carbon Oxide in contrast to the Silicon Dioxide atomic lattice?
In Minecraft silicon_dioxide can be obtained from sand, quartz, end_stone, blockGlass, cobblestone, stoneGranite, stoneDiorite, Magma, stoneAndesite, stone.
Quartz ware
Crystalline silica is called a quartz. When melting, silica softens gradually and gradually solidifies with cooling. Quartz expands very little when heated. Therefore, quartz dishes can be heated to white, cast into cold water and it does not crack.
River sand
Silicon oxide refers to acid oxides. It does not combine with water. The rivers flow for centuries through the channel of white sand.
Silicic acid
Silicic acid precipitates as a gelatinous precipitate. The formal formula is H2SiO3. The structural formula is a little more complicated. This is a chain of four or more silicon atoms, each of which is enclosed in a tetraider of 4 oxygen atoms. Silicic acid is a very weak acid. Silicon acid salts are called silicates. Alkali metal silicates are called soluble glasses.
How to get salts of silicic acid?
Glass
The glass is made of white sand, limestone and soda, by melting their mixture.
In Minecraft it’s enough just to burn the sand in the oven to get the glass.
The main property of any glass is that it passes from a liquid state to a solid state not abruptly, but thickens as it cools down to solidification. This property is based on the glass blowing business, the production of sheet glass and glass fiber.
Sheet glass (or blocks) can be made in cannery by prescription by installing 6 glass blocks on a workbench.
By introducing into the glass, instead of the basic oxides of sodium and calcium, oxides of other mono- and bivalent metals, instead of acidic silica (IV) – boron oxide, it is possible to vary glass properties in a wide range.
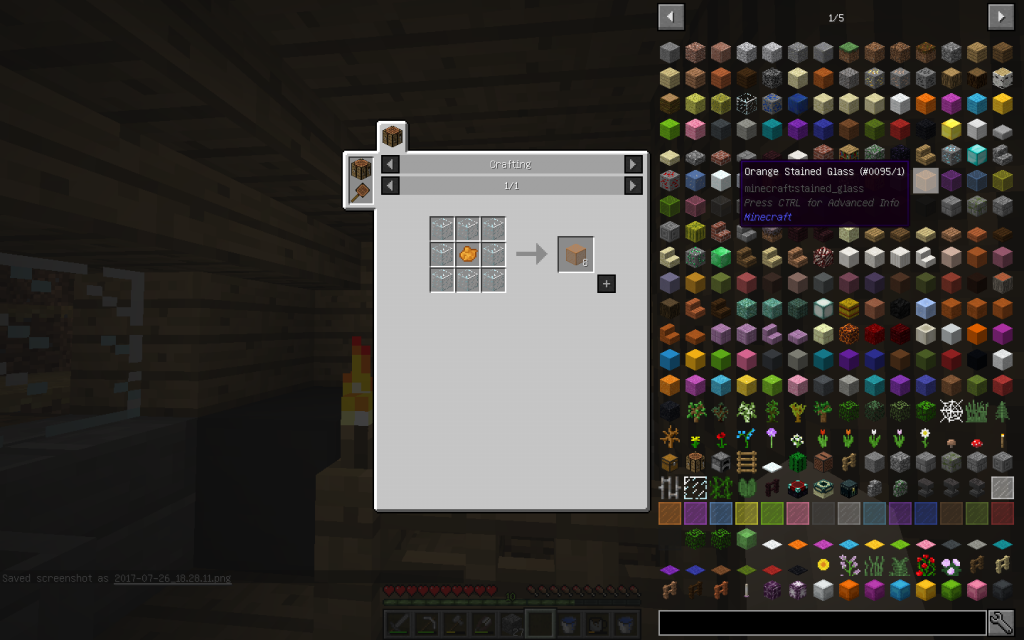
Colored glasses are obtained by introducing into the starting mixture compounds of such metals, of which the yon possesses a given color. The following additives change the color of the glass.
Nickel – blue-black glass.
Iron oxide (II) is a blue-green glass.
Together with chromium – a rich green color.
Sulfur and selenium, together with iron salts, etc., is used to form polysulfides of iron, zinc, cadmium and get amber glass, as well as colors from yellowish to almost black.
Manganese is a purple glass.
Selenium with sulphide Cadmium – Ruby glass.
Potash – blue glass.
Tin oxide – milk glass.
Copper oxide – the turquoise color of glass.
Chrome is an emerald green color.
Gold is the color of the cranberry.
Silver is an orange-red color.
In cannone, glass and other objects can be painted with the help of an Atomic Reconstructor with a special color lens. In this way, the Atomic Reconstructor, when changing the color of the glass, must change the type of impurity ions.
Or the same effect can be obtained by melting the glass. In this case, the thing will lose its shape. After making the additive and cooling the glass, giving the original shape.
Polymorphism
Silicon oxide has several polymorphic modifications. Α-quartz, β-quartz, tridymite, cristobalite, opal, chalcedony, quartz, lutecite, authigenic quartz, coesite, stishovite – hexagonal quartz.
Answer
pi – bond
2NaOH + SiO2 = Na2SiO3 + H2O