Protein can be obtained from threads of spider and flax, from wool, condensed Milk (which is obtained in Evaporator) and egg. To get more elements and their variety, it is better to lay more items.
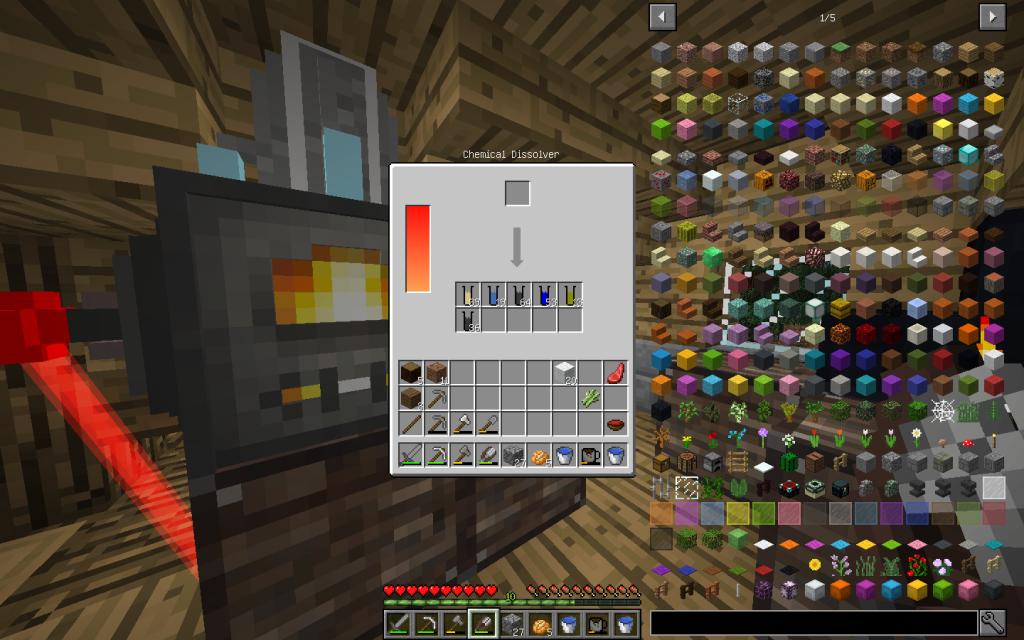
From each block of wool, two proteins are obtained, regardless of the color of the coat. Further decomposition is best done by laying 4 protein substances in Chemical Dissolver, because it is more likely to get sulfur out and more other simple chemical elements.
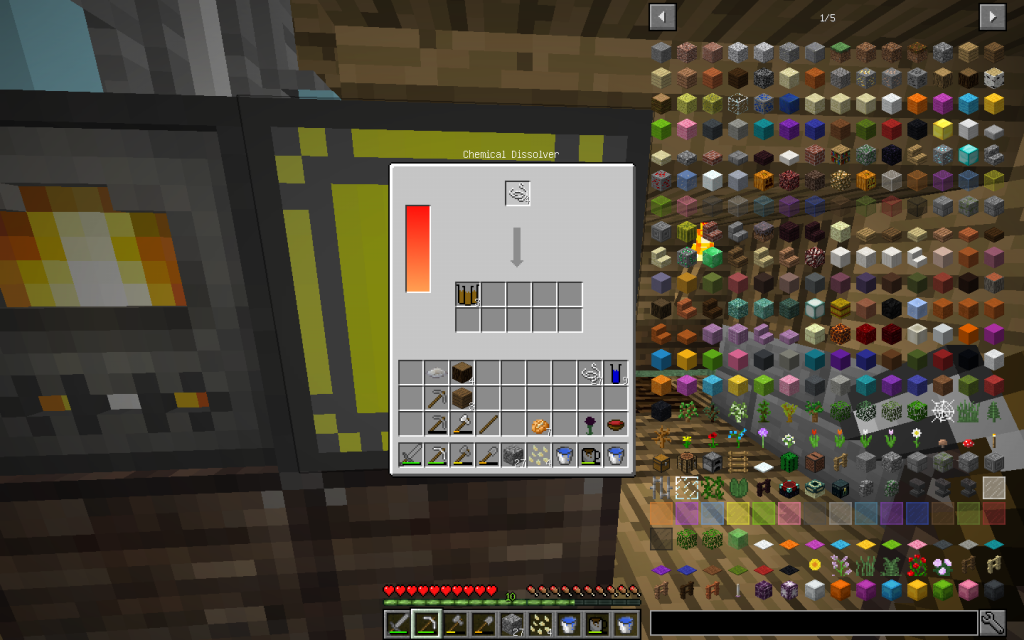
In the top line, what happens if you lay only one Protein in the Chemical Dissolver. The lower yield of 4 Proteins. As you can see, we also got Sulfur (16th serial number in the periodic table of Mendeleyev and 3rd row).
- SETUP NEW MINECRAFT CLIENT FOR MINECRAFTFORGE 1.12 ON YOUR COMPUTER
- MINECHEM v6
- ALCHEMISTRY V 0.6.1 & ACTUALLY ADDITIONS
- DIAMOND
- NETHER FORTRESSES
- CHEMICAL MACHINES
- CELLULOSE
- MEDIUM STORAGE CRATE
- SUCROSE
- PROTEIN
Chemistry Zumdahl, 7 ed. Houghton Mifflin Company Boston New York
Of 16 proteins or 8 blocks of wool, more than one stack of Carbon is produced. When we accumulate 8 stacks of Carbon, it will be possible to synthesize yet another artificial diamond.
Proteins are very large molecules, they have a spatial structure on 4 levels of internal hierarchy. To support the structure in the proteins work 5 types of chemical bond (page 1025-1031 of a chemical textbook). These are the following relationships: Ionic, hydrogen bonding, covalent, London dispersion, and dipole-dipole.
How called shape of the protein?
In Chemical Dissolver, we got Sulfur. Because the amino acid cysteine plays an important role in proteins. Cysteine forms an S-S connection, called disulfide linkage. These connections, because of the possibility of shifting the SH groups (exchanging the places of neighboring atoms), allows the proteins to change the spatial configuration.
These bonds play an important role in denaturation. For example, when we prepare the Egg, its protein from the transparent turns white – this is the process of protein denaturation under the influence of external energy (temperature). If we separate the protein from the yolk and beat it with sugar – then we will get a bisque cookie – which is the same as the protein denaturation product but under the influence of mechanical energy. Radiation also promotes protein denaturation. You can also destroy the protein with a laser (which is used in the method Mass Spectral Analysis of the Protein on an Acid Matrix – page 87 of a chemical textbook).
If you make the laser beam not so powerful and destructive, then you can use a laser to assemble the protein in a suitable dipping (enzymes).
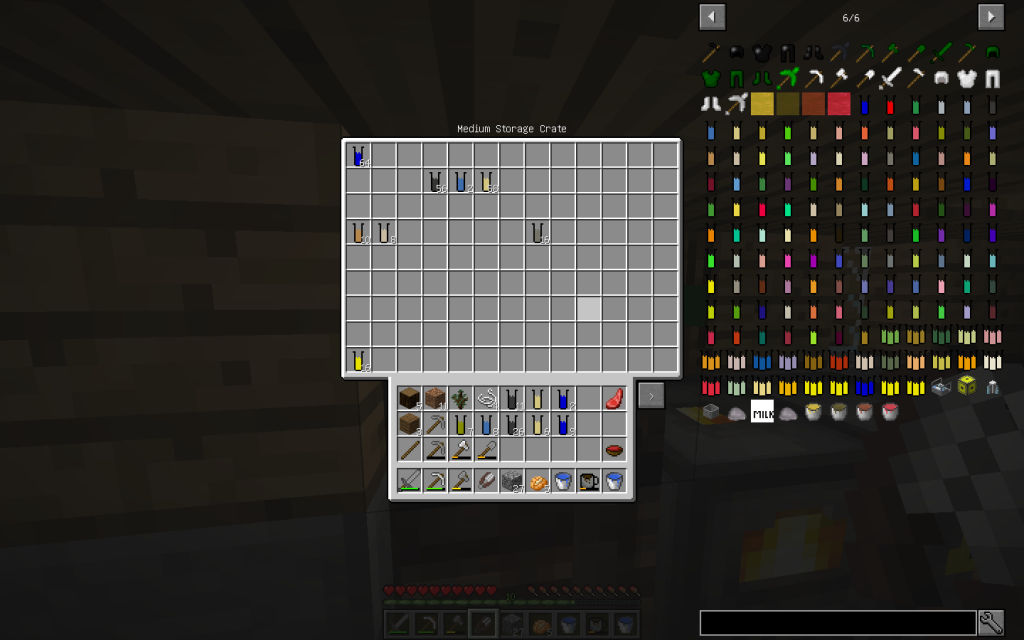
Let’s put four Blocks of Wool or String in the Chemical Dissolver – we got 8 volumes of complex Protein.
One will be placed in our collection, and the rest will again be decomposed in a Chemical Dissolver. We got the substances known to us and Sulfur. Pay attention that we got a lot of Carbon. If you have accumulated more than one stack of Carbon, keep the surplus in the second compartment of our Vault for future use.
What is the formal Cysteine formula?
Proteins perform the most important functions in the organisms of living beings. For example, in Polar latitudes, fish that contain blood are not present red blood cells and hemoglobin (page 756, 516, of a chemical textbook). Therefore, in the blood of this fish, at low temperature, crystalline crystals are not formed. And the blood itself is white.
The hemoglobin protein resembles 4 tangles collected in a tetraider. The size of each coil of protein is of the order of 100-1000 atomic sizes, which is very much for one molecule. These balls have the property of gathering together, and even against the force of gravity.
Apparently this happens because the coils themselves form, as it were, the plates of the condenser, which are attracted to each other. Moreover, electrons can spontaneously resonantly arise under the action of cosmic rays and resonance conditions.
Reactions in the Proteins occur with the help of Enzymes (pages 562-563, 557-558, 973-979 of a chemical textbook).
What are the names of substances that accelerate the reaction within the body of living beings?
Answer
Tertiary structure
C6H12N2O4S2
Enzymes