- SETUP NEW MINECRAFT CLIENT FOR MINECRAFTFORGE 1.12 ON YOUR COMPUTER
- MINECHEM v6
- ALCHEMISTRY V 0.6.1 & ACTUALLY ADDITIONS
- DIAMOND
- NETHER FORTRESSES
- CHEMICAL MACHINES
- CELLULOSE
Chemistry Zumdahl, 7 ed. Houghton Mifflin Company Boston New York
Preparation for chemical experiments
In order to successfully carry out experiments, one must understand the interrelation between the chemical and physical properties of similar elements. Russian scientist-chemist Dmitry Mendeleev more than 100 years ago discovered the principle of placing chemical elements on the basis of relative atomic mass.
Later researchers were able to propose a model of the chemical atom and experimentally proved the dependence of the properties on the order number of the element which is equal to the charge of the atomic nucleus.
Review the pages of the textbook and keep the Periodic System of Mendeleyev’s chemical elements at hand (pages 299-301, 55-57, 875-876 of a chemical textbook). In the system, chemical elements are grouped according to their properties. If we know how the model of each atom is constructed, then it is possible to determine the chemical and physical properties of a pure substance, solution, mixture or complex substance on the basis of a model of a molecule (several atoms combined into a common structure).
We need (we need to take in the kitchen): a teaspoon of starch on a saucer, a match and a magnifying glass.
Task: Toss the match and consider the broken tip of the match under the magnifying glass. You see the fibers.
Look at the starch with a magnifying glass – you see the crystals.
What are the lines of the periodic table of Mendeleyev called?
Let’s start the experiments
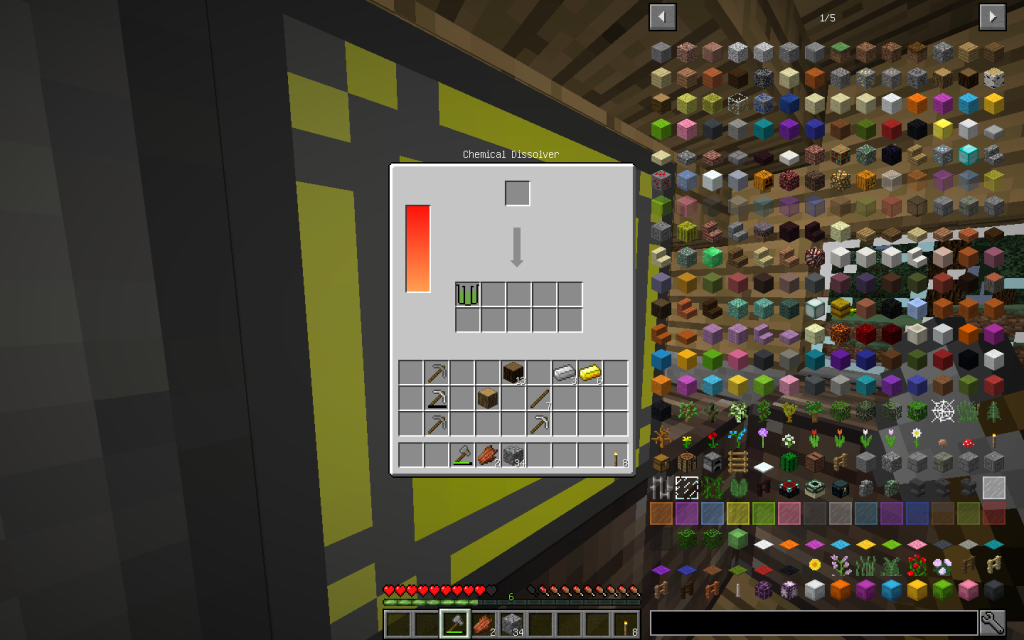
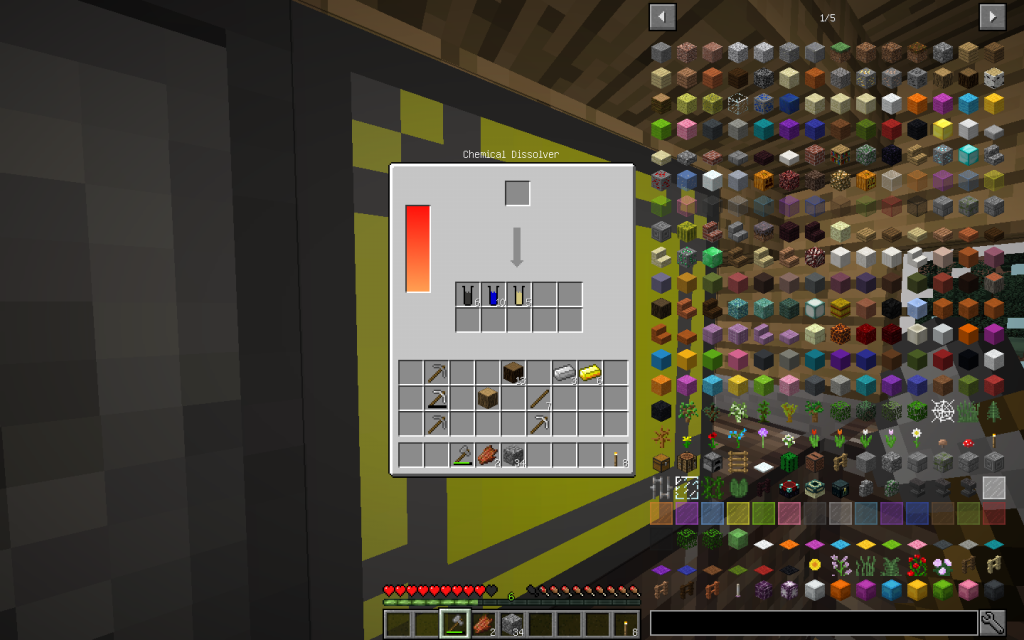
Let’s start our chemical experiments. We will place one log (one cubic meter) of Oak wood in the Chemical Dissolver. We instantly receive cellulose. Starch and cellulose can be obtained from wheat.
Cellulose (pages 1034-1035 of a chemical textbook) is an important product of industrial production based on wood. Further, it is usual to make paper of different quality from cellulose. Cellulose and Starch have the same formula but different molecular models. Therefore, these substances look different, although they consist precisely of the same number of simple chemical elements.
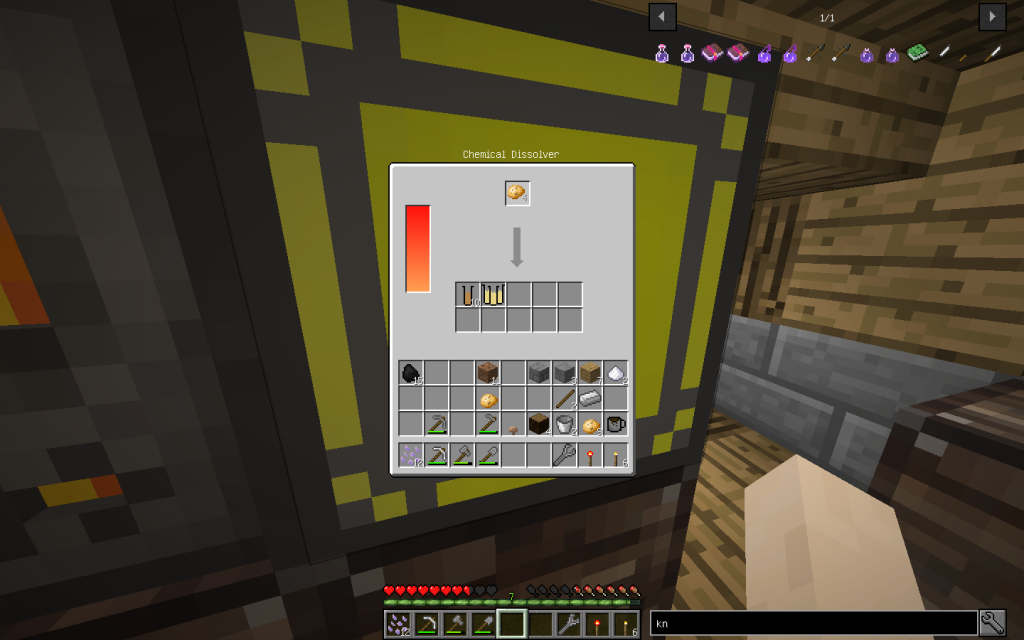
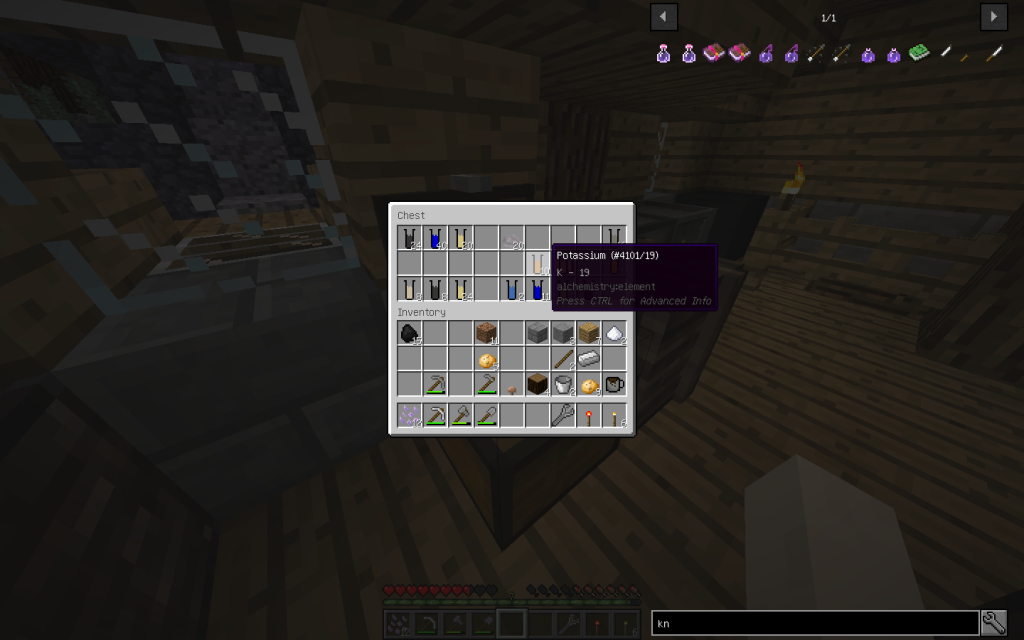
Starch can be obtained from potatoes sometimes together with potassium.
You need to put 4 potatoes into the Chemical Dissolver, not less. It turns out 2 times more moles than from a tree, sometimes in addition 2 moles (total 10 tubes) of potassium.
Starch (C6H10O5)n — the mixture synthesized by plants under the influence of light during photosynthesis has the same formula as cellulose. The letter n means that this formula can be repeated many times and form a long molecule, which in turn form long fibers. A tree consists of such fibers.
A chain of what substance is packaged in plant proteins (like Starch) for energy storage?
One medium potato contains about 800 mg of potassium.
Cellulose is a natural polymer. That is, a substance that consists of monomeric units (C6H10O5)n – combined into long macromolecules. Structures (fibers) from macromolecules can be seen with an unaided eye. Therefore, they are called macromolecules. That is, large molecules.
Place Cellulose in Chemical Dissolver.
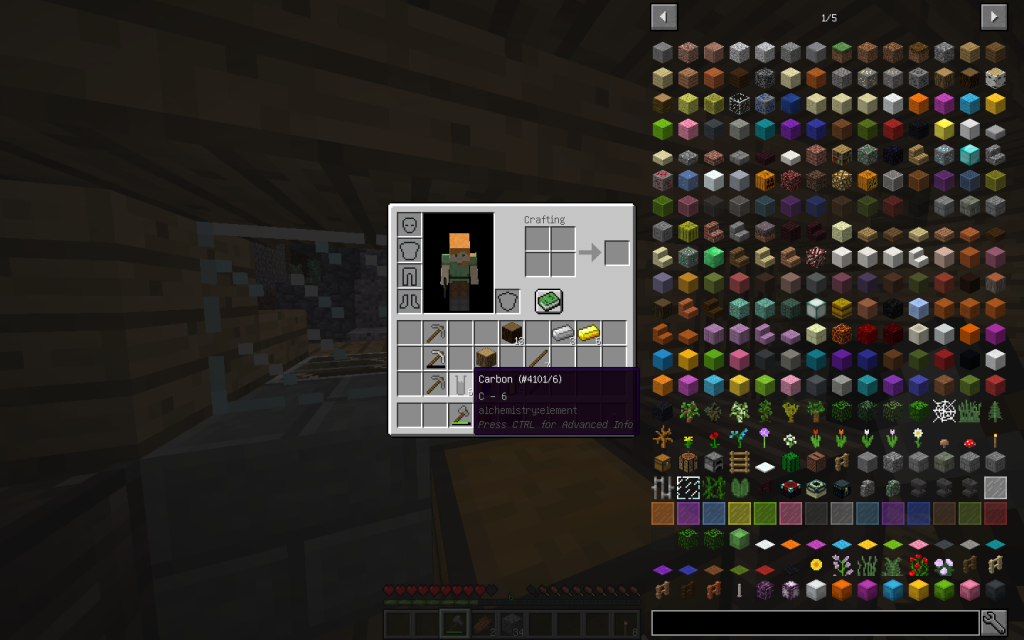
From one cellulose (C6H10O5)n we get 6 Moles of carbon (6th ordinal number in the Mendeleyev System),
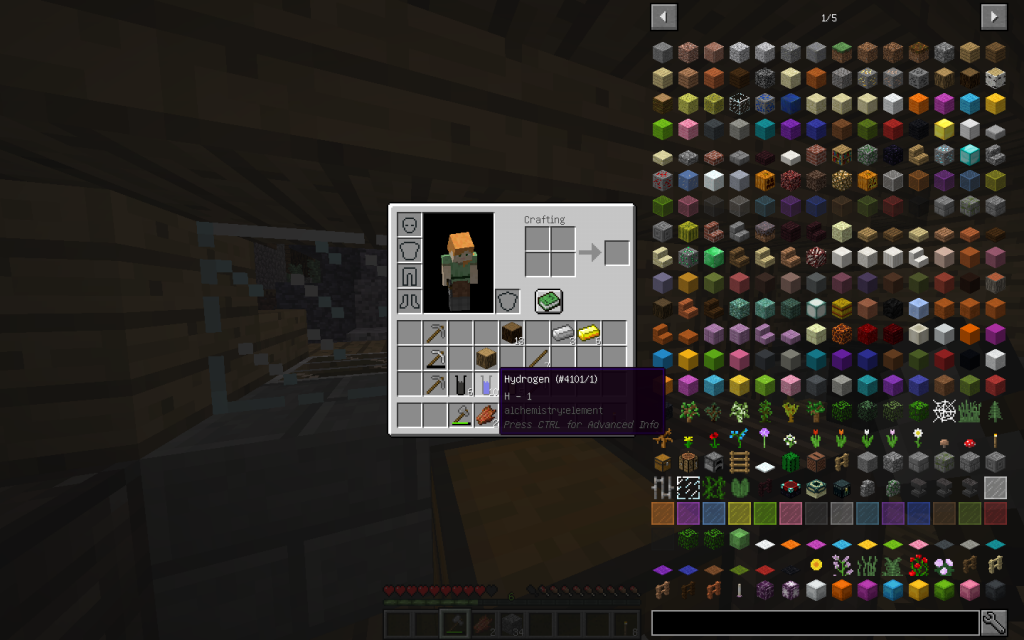
10 moles of hydrogen (the very first element in the periodic table and the most common in the universe, in the Universe there are giant hydrogen nebulae)
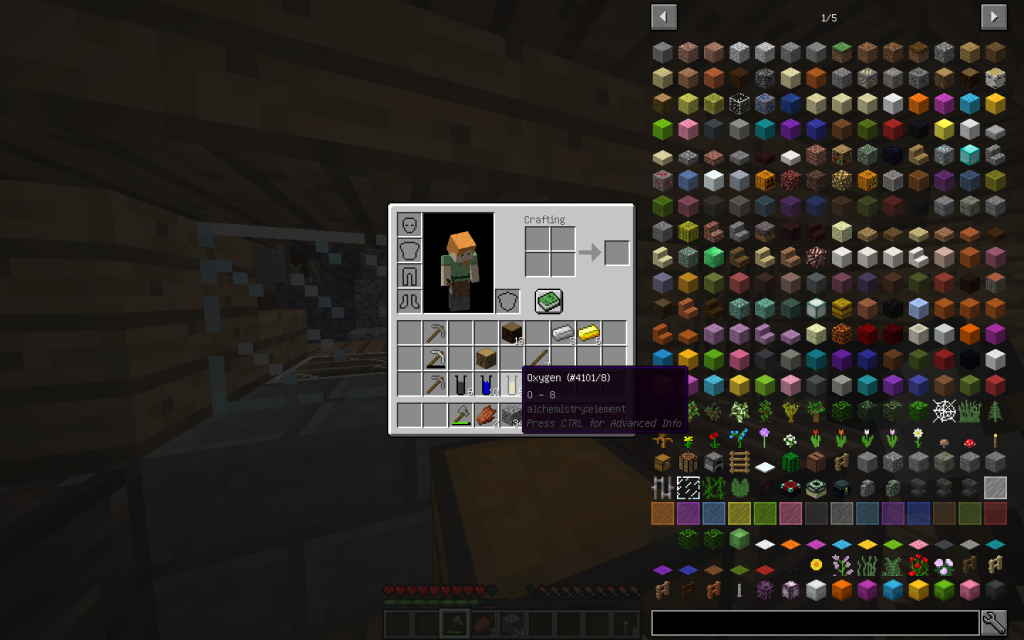
And 5 moles of oxygen (8 in the periodic table). What exactly corresponds to the chemical formula (C6H10O5)n. Where individual moths of a simple substance are packaged in one mole of a monomeric unit.
Cellulose in the form of wood is used in the construction and manufacture of household items. Cotton, linen and other fibers are used for making yarns and spinning in the textile industry, which produces clothing and materials and things used in everyday life.
Supplement (links to future experiments)
Paper is a thin layer of fiber fibers. Wood is 50% fiber. There are chemical methods for purifying wood fiber from impurities such as resins. With a sulphide purification method, waste is formed – sulfide liquors, if not processed, they strongly pollute nature. They are used to produce Ethyl alcohol.
Answers
Rows and Periods
Glucose