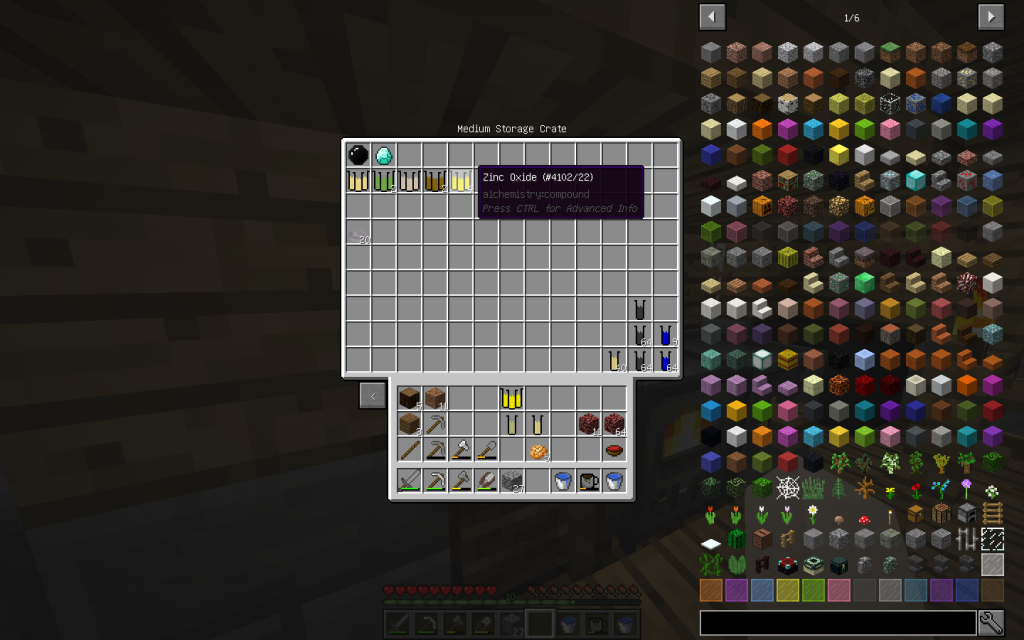
Zinc oxide (zinc oxide) ZnO is a colorless crystalline powder, insoluble in water, yellowing upon heating and subliming at 1800 ° C.
When heated, the substance changes color: white at room temperature, zinc oxide turns yellow.
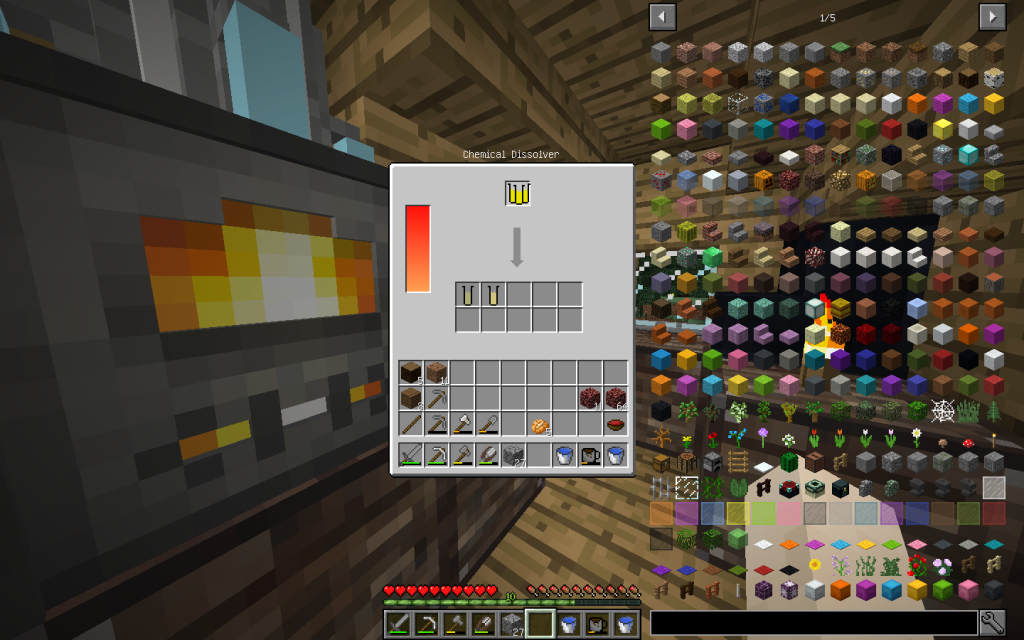
In Minecraft Zinc Oxide can be obtained from Netherrack or by reacting pure zinc from mieralSalt (Which is obtained with Evaporator from ordinary water and subsequent decomposition into Chemical Dissolver) with oxygen in the Chemical Combiner.
- SETUP NEW MINECRAFT CLIENT FOR MINECRAFTFORGE 1.12 ON YOUR COMPUTER
- MINECHEM v6
- ALCHEMISTRY V 0.6.1 & ACTUALLY ADDITIONS
- DIAMOND
- NETHER FORTRESSES
- CHEMICAL MACHINES
- CELLULOSE
- MEDIUM STORAGE CRATE
- SUCROSE
- PROTEIN
- SILICON
- SILICON DIOXIDE
- ZINC OXIDE
Chemistry Zumdahl, 7 ed. Houghton Mifflin Company Boston New York
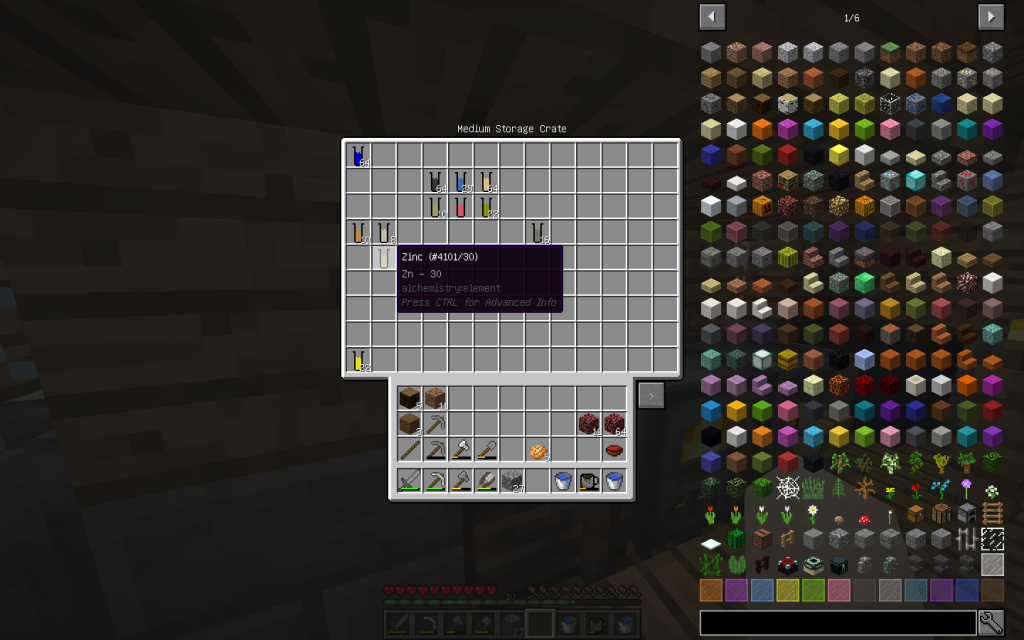
In Chemical Dissolver, we decompose this substance into Zinc and Oxygen.
Place the resulting Zn (pages 305, 815, 947, 955, 982, 60 of a chemical textbook) in its place.
What the orbitals configurations of the transition metal Zn?
Zinc is a metal resistant to corrosion, so it is used in the production of galvanized roofing iron. In the Periodic System of Chemical Elements, zinc is located beneath Calcium in the 5th row and in the 2nd group.
In which series (subgroup) of metals is Zinc?
Semiconductor
Zinc oxide is a direct-gap semiconductor with a band gap of 3.36 eV. Natural doping with oxygen makes it an n-type semiconductor.
To radiation and create blue LEDs, Zinc_oxide is well suited. Thin films and other nanostructures based on zinc oxide can be used as sensitive gas and biological sensors.
Recently, interest in zinc oxide (ZnO) has increased significantly due to the fact that this material is considered as one of the promising for the creation of light-emitting structures and lasers of the blue and ultraviolet spectral region. In addition, zinc oxide is a traditional low-voltage cathodoluminophore with a green glow, used in a variety of optoelectronic display devices.
Answer
Zn: [Ar] 4s23d10
In the first row is the Transit of metals at the very end. Or at the end of the sub-group of the 4th period.