Let’s begin our study of the nature of chemical reactions using the traditional device for each alchemist.
Evaporator
This is the Evaporator. In the picture below under Electrolyzer. Saucer with salt water from a practical task is a small model that works like a real Evaporator. Sometimes, to accelerate the evaporation of water to the Evaporator, energy is supplied through the heat carrier. For example, put a bath in a jar with hot water and put a fire under the vessel or install any other thermo-elements.
In the current version of Mod, it is possible to use raw water or milk, which can be brought with metal buckets, as a raw material for it.
It is possible to add water through the remote interface to the water intake point in the pond near the house.
- SETUP NEW MINECRAFT CLIENT FOR MINECRAFTFORGE 1.12 ON YOUR COMPUTER
- MINECHEM v6
- ALCHEMISTRY V 0.6.1 & ACTUALLY ADDITIONS
- DIAMOND
- NETHER FORTRESSES
- CHEMICAL MACHINES
- CELLULOSE
- MEDIUM STORAGE CRATE
- SUCROSE
- PROTEIN
- SILICON
- SILICON DIOXIDE
- ZINC OXIDE
- MINERAL SALT
- POTASSIUM NITRATE
Chemistry Zumdahl, 7 ed. Houghton Mifflin Company Boston New York
(pages 445-446, 890-892, 46-47, 876-878, 455 of a chemical textbook)
Practical task: Take a glass of water (preferably warm) and dissolve in it as much salt as possible. As you will notice the water will remain colorless and the crystals of the soap will not be visible. If you take the most precise filter and filter the water, the dissolved salt (salt ions) will remain in the water, because the crystals of the salt have broken up into separate ions and are located between the molecular chains of water.
Now take a saucer or any small dishes and pour water into it with dissolved salt and put it in the sun or in a warm place. (It can be put for a few seconds in a microwave oven, provided that the dishes are not metal but glass, for example). The next day or a few hours you will see (in the microwave oven after 20 seconds) that the water evaporated and the crystal formed salt in the saucer.
Acids
Hydrogen is the most common chemical element in the universe. This was determined by astronomers due to the spectral analysis of light from stars and nebulae in space.
Hydrogen compounds include acids. In nature, you can find many acids: citric acid in lemons, malic acid in apples, oxalic acid in the leaves of sorrel. Ants defend themselves from enemies by producing Formic acid. Formic acid is found in bee venom and stinging nettle hairs.
When the grape juice turns sour, we get Acetic acid. And when milk turns sour – lactic acid. Lactic acid is obtained when cabbage is sour.
Acid is a complex chemical substance, in each molecule of which there is one or more hydrogen atoms and an acid precipitate.
Sulfuric acid
There are also industrially produced acids – Sulfuric acid H2SO4. When you get into Sulfuric acid water – a lot of heat and splashes. This acid is dangerous for the human body. Sulfuric acid absorbs moisture and air from other gases. Charred wood, leather, fabric.
Hydrochloric acid
HCl
Aqueous solution of Chlorine Hydrogen which has a sharp odor. The chemical reaction between Zinc and Hydrochloric acid can be used to produce Hydrogen and Zinc Chloride ZnCl2.
Zn + 2HCl = ZnCl2 + H2
In this case, the Zn atoms of the Zn (divalent metal) replace the Hydrogen H atoms in two molecules of Hydrochloric acid 2HCl to form the ZnCl2 Zinc chloride salt. This salt is contained in the sediment Mineral Salt, which remains after evaporation of water in the Evaporator.
Salt is a complex chemical substance in which metal atoms are bound to an acid residue.
The name of salts is formed from the Latin name of acid residues.
Acidic residue
Hydrochloric acid Cl is called chloride.
Sulfuric acid SO4 – sulfate,
Nitric acid NO3 – nitrate,
Phosphoric acid PO4 – phosphate
Composition of Mineral Salt
Immediately after placing the water in the installation by right-clicking a filled bucket of water, the evaporation process begins, leaving a mineral deposit of water. Which is called Mineral Salt.
Mineral Salt is a very useful substance that contains important metals and salts. In Minecraft Mineral Salt composition is: sodium_chloride, lithium, potassium chloride, iron, copper, zinc.
Two salts of Chloride and three metals.
Name of Acid and Salt
| Acids | Formula | Salt |
| Hydrofluoric | HF | Fluorides |
| Hydrochloric | HCL | Chlorides |
| Hydrobromic | HBr | Bromides |
| Hydrogen iodide | HI | Iodides |
| Sulfuric | H2SO4 | Sulphates |
| Hydrogen sulfide | H2S | Sulphides |
| Nitric acid | HNO3 | Nitrates |
| Phosphate | H3PO4 | Phosphates |
| Carbonate | H2СO3 | Carbonates |
| Silicon | H2SiO3 | Silicates |
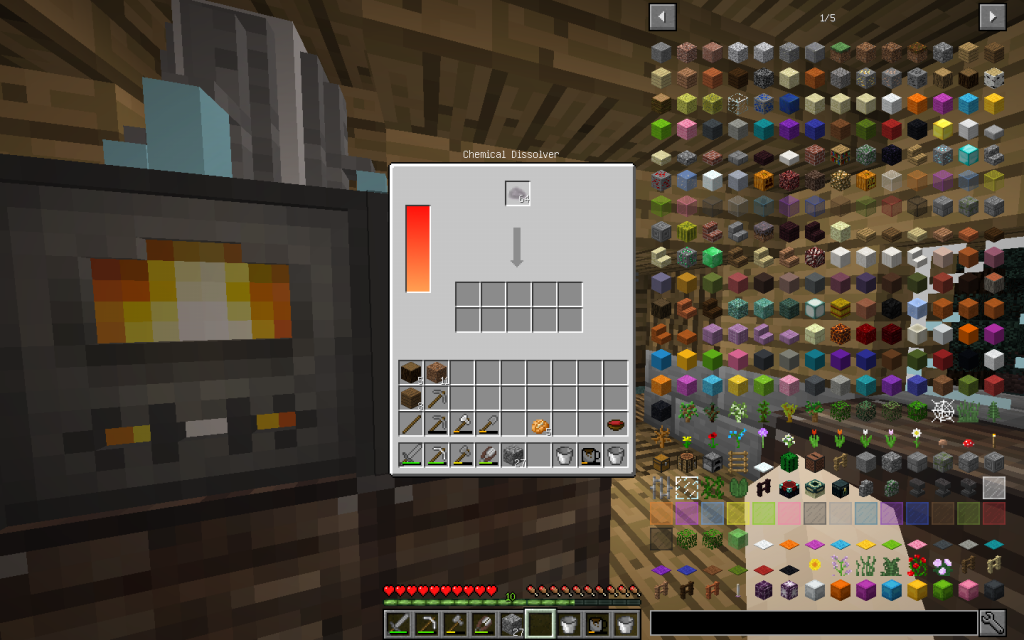
Getting chemical elements and chemicals from Mineral Salt
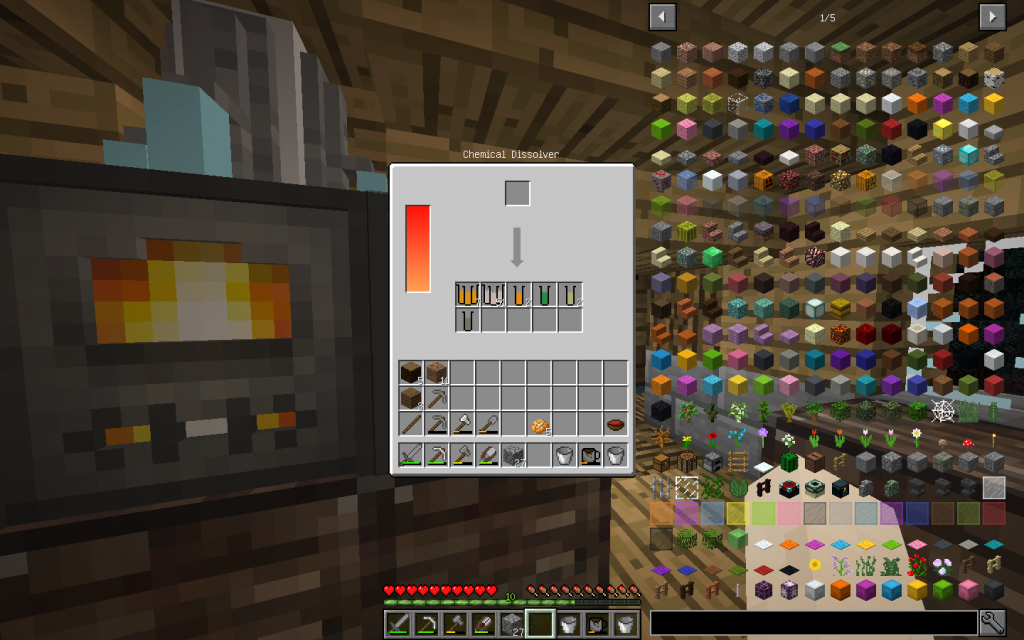
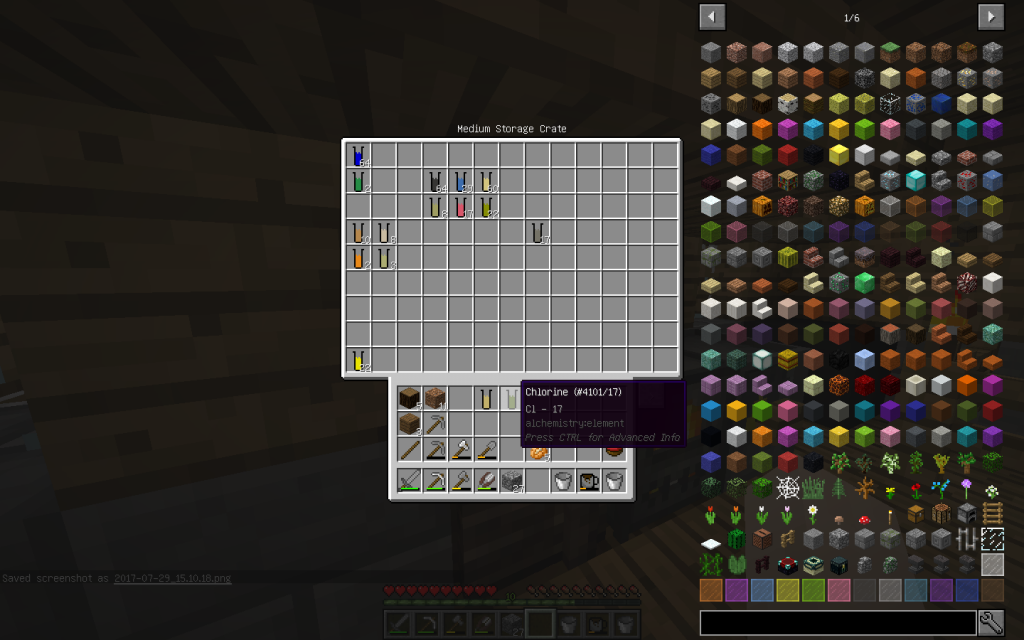
Let’s put Mineral Salt in Chemical Dissolver. It is better to immediately use 64 stacks of Mineral Salt, as it is more likely to get all possible chemical elements. If you use a smaller amount, then probably some materials will not be received.
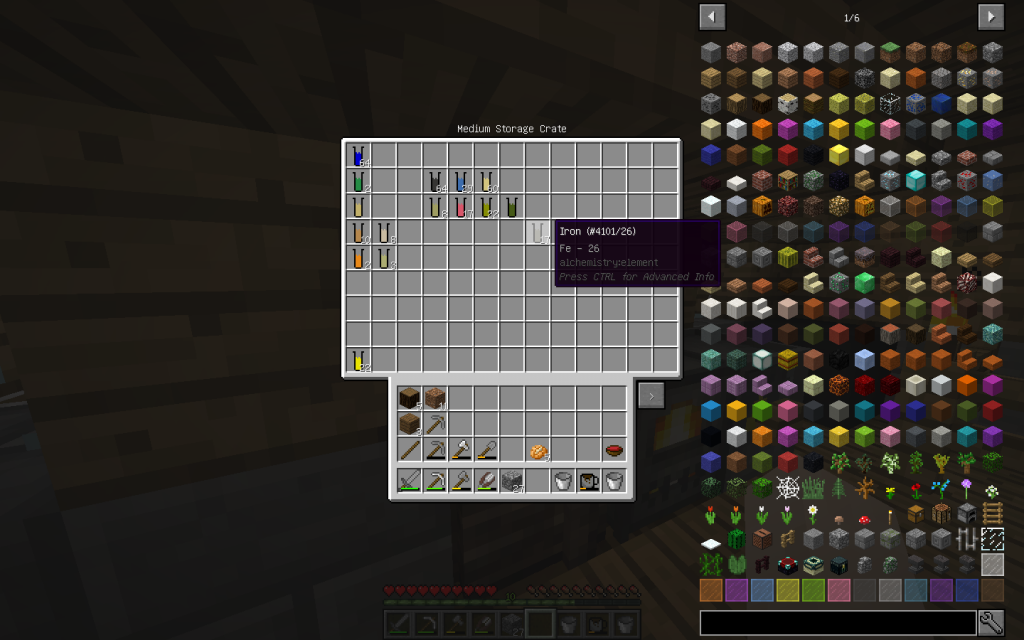
From the stack of Mineral Salt, we obtained 7 moles of Potassium Chloride, 51 moles of Sodium Chloride, 2 moles of Copper (Cu-29), 1 Mole of Lithium (Li-3), 2 moles of Zinc (Zn-30), 1 mole of Iron (Fe-26 ). The quantitative composition of the solution can be different. But the chemical elements in the water of Minecraft are found only these.
Periodic system of chemical elements
Place all the elements in their places in the Periodic system of chemical elements.
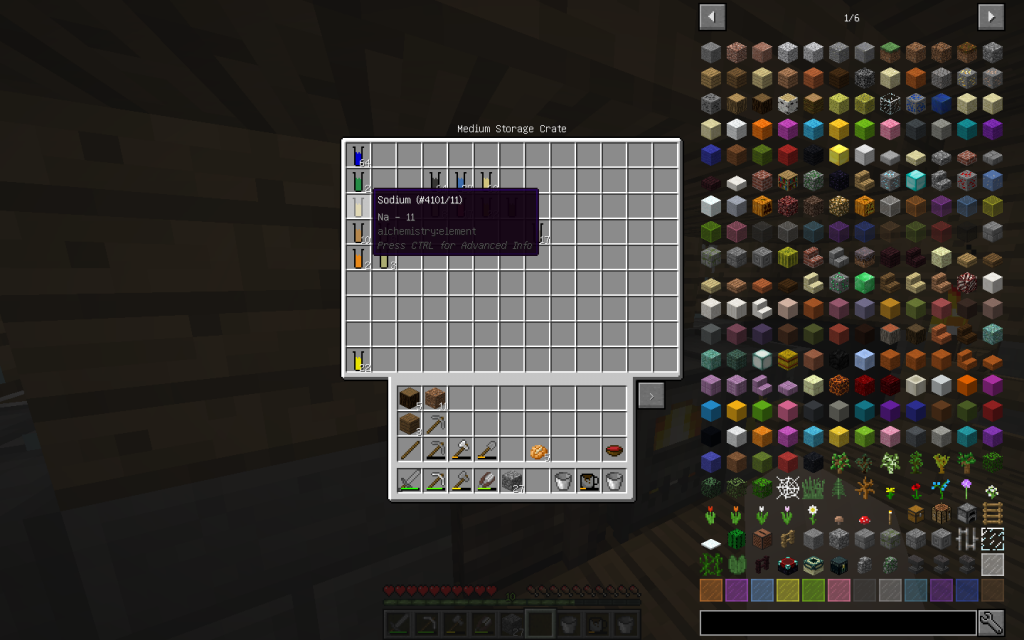
Sodium Chloride (pages 154-159, 456-458 of a chemical textbook) is ordinary table salt that we add to our meals. Let us decompose this substance into its constituent elements. To do this, use Chemical Dissolver. We obtained 1 mole of Sodium (Na-11) and 1 mole of Chlorine (Cl-17).
(pages 53-55, 316-318, 456-458 of a chemical textbook)
What is an ion?
Between metals and acid residues, the bond is ionic. The metal ion of Sodium (Na-11) is positively charged. Hydroxide-Chlorine (Cl-17) is negatively charged.
Which element and in which column (Group) is Chlorine?
Lithium (Li-3), Sodium (Na-11), Potassium (K -19 which we got from potatoes, and also can be obtained from Potassium Chloride) are all Alkali metals and therefore are in the first column of our Periodic Table. Or what is the same in the First Group of the Periodic Table.
Copper (Cu – 29), Silver (Ag – 47), Gold (Au – 79) – also are in the first column, but belong to an auxiliary subgroup with d electronic orbitals, which fill at a nonvalent level (they fill at the level previous row). But these elements exhibit a valence one. Like the metals of the First group (the first column).
Structure
Sodium chloride forms colorless crystals of cubic syngony, space group Fm3m, cell parameters a = 0.563874 nm, d = 2.17 g / cm3. The Sodium Chloride Crystal (NaCl) consists of two face-centered cubic sublattices shifted by half a period.
Potassium Chloride
(pages 53-55, 316-318, 880-882, 729, 735-736 of a chemical textbook)
The potassium salt of hydrochloric acid is KCl. White matter without odor. Structure by the type of Sodium Chloride (NaCl). White crystalline substance without a smell.
In laboratory conditions, potassium chloride can be obtained by reacting potassium hydroxide with hydrochloric acid:
KOH + HCl = KCl + H2O
Crystals of potassium chloride may form holograms. The injection of a solution of potassium chloride is deadly to humans.
In Minecraft, Potassium chloride can be obtained from mineralSalt, stoneGranite, stoneDiorite, Magma, stoneAndesite, stone.
Potash fertilizers
In Solikamsk in Russia in the 1920s, the largest Silvinit deposit (billions of tons) was found at a depth of 200-300 meters. Silvinit is a fused crystal of potassium chloride (KCl) and sodium chloride (NaCl).
How to separate potassium chloride from sodium chloride (pages 980-983 of a chemical textbook)?
For potato plants, grapes, tobacco and other chloride ions are not useful. Therefore, Potassium chloride is introduced from the fall in the hope that chlorine ions will be washed by spring spring waters. But it is better to replace Potassium Chloride with Potassium Phosphate or Potassium Nitrate.
An excellent and qualitative Potash fertilizer is ash from the burning of plant materials. In addition to potassium carbonate K2CO3, there are phosphorus compounds P and microelements.
Answers
Ion is an atom or group of atoms that has a net positive or negative charge.
Near Sere (S-16) in the 7th group and 3rd row.
The solubility of potassium chloride decreases sharply with decreasing temperature. Therefore, when the solution of Silvinit saturated to 100 ° C is cooled to room temperature, a significant part of the potassium chloride falls out of the solution. The crystals are separated by filtration. Another way of separating salts is called flotation.